veriseq nipt v2
VeriSeq NIPT Solution Package Insert Translated into Czech. 2 days agoVeriSeq NIPT Solution v2.
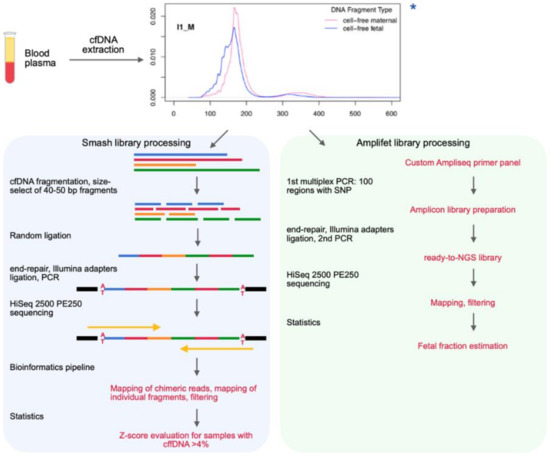
Genes Free Full Text Nipt Technique Based On The Use Of Long Chimeric Dna Reads Html
Product includes components of library preparation sequencing and analysis.

. The new version expands the range of chromosomal and sub-chromosomal conditions associated with birth defects that laboratories can screen for. VeriSeq NIPT Solution v2 Package Insert Translated into Brazilian Portuguese. The automated in-lab IVD solution will enable NGG Thailand to introduce the Qualifi Prenatal Test.
Instructions for using the VeriSeq NIPT Solution v2. Enhanced Time Savings and Sample Integrity. The integrated VeriSeq NIPT Solution v2 provides every - thing needed to run the assay.
Created edited and updated the Package Insert and Software Guide for the product. Instructions for processing samples with the VeriSeq NIPT Solution v2. The VeriSeq NIPT Solution v2 assay enables accurate identification of fetal aneuploidy allowing detection of genome-wide fetal chromosomal anomalies with high clinical sensitivities and specificities and a low assay failure rateClinical Trial Notification CTN identification number ID.
Equipment Height Width Depth Weight VeriSeqOnsiteServerv2 438 cm 173 in 178 cm 7in 635 cm 25 in 259kg 57lbs VeriSeqNIPT MicrolabSTARwithAutoload 903 cm 356 in 199 cm 783 in 1006 cm 396 in 160kg 353lbs VeriSeqOnsiteServerv2PlacementRequirements PositiontheVeriSeqOnsiteServerv2toallowfor. All Reproductive Health Products. Instructions for processing samples with the VeriSeq NIPT Solution kit.
This noninvasive test provides an option to screen for aneuploidy in all autosomes chromosomes X Y and partial deletions and duplications greater than 7 Mb across the genome. All Reproductive Health Products. Use teh BWA Enrichment app or the Isaac Enrichment app v20 or v21 for analysis.
The VeriSeq NIPT Solution v2 is an in vitrodiagnostic test intended for use as a screening test for the detection of genome-wide fetal genetic anomalies from maternal peripheral whole blood specimens in pregnant women of at least 10 weeks gestation. PDF 1 MB Aug 13 2021. VeriSeq NIPT Solution v2 Package Insert 1000000078751 v06 1 MB.
VeriSeq NIPT Solution v2 provides accurate information about fetal chromosomal status as early as 10 weeks of gestation using a single maternal blood draw. At Illumina our goal is to apply innovative technologies to the analysis of genetic variation and function making studies possible that were not even imaginable just a few years ago. Hands-free throughput and sample integrity are enabled through powerful features such as eight.
VeriSeq NIPT Solution Consumables Equipment List Translated into Brazilian Portuguese. PDF 1 MB Feb 5 2022. The VeriSeq NIPT Solution v2 is an in vitro diagnostic test intended for use as a screening test for the detection of genome-wide fetal genetic anomalies from maternal peripheral whole blood specimens in pregnant women of at least 10 weeks gestation.
VeriSeq NIPT Solution v2 Package Insert 1000000078751 v06 PDF 1 MB Aug 16 2021. VeriSeq NIPT Solution v2 Package Insert 200006957 v00 for Canada. VeriSeq NIPT Solution v2 is a CE-IVD approved and next-generation sequencing NGS-based method to noninvasive prenatal testing NIPT.
VeriSeq NIPT Solution v2 uses whole-genome sequencing to detect partial duplications and. VeriSeq NIPT Solution v2. The VeriSeq NIPT Solution v2 is an in vitro diagnostic test intended for use as a screening test for the detection of genome-wide fetal genetic anomalies from maternal peripheral whole blood specimens in pregnant women of at least 10 weeks gestation.
Served as technical writing project lead for the release and launch of VeriSeq NIPT Solution v2 an end-to-end solution for non-invasive prenatal testing. VeriSeq NIPT Solution Package Insert Translated into Danish. VeriSeq NIPT Solution Package Insert 1000000001856 v08 1 MB.
1000000067940v05 Settembre 2020 AggiunteleistruzioniperlenuovefunzioniCodificadelbackupe. VeriSeq NIPT Solution Consumables Equipment List Translated into Bulgarian. To set up the run use the FASTQ workflow and stream the run into BaseSpace.
The assay provides information about fetal chromosomal status as early as 10. The BaseSpace RNA-Seq Alignment App analyzes data from the TruSight RNA Pan-Cancer Panel providing. VeriSeq NIPT Solution v2 Package Insert Translated into Brazilian Portuguese.
After analysis download VCF files from BaseSpace and into the VariantStudio software to report and filter variants. Comprehensive IVD in-lab aneuploidy screening solution for accurate NIPT results in 26 hours. Cronologiarevisioni Documento Data Descrizionedellamodifica Documenton.
Yes BaseSpace can be used to analyze data from TruSight Cardio runs. Instructions for analyzing assay data using the VeriSeq NIPT Solution v2 software. Comprehensive IVD in-lab aneuploidy screening solution for accurate NIPT results in 26 hours.
VeriSeq NIPT Solution Consumables Equipment List 1000000076886 v03 ZIP 1 MB Apr 12 2021. PDF 1 MB Feb 5 2022. Chapter1VeriSeqNIPTSolutionv2 Introduction 1 SystemArchitecture 2 Introduction TheVeriSeqNIPTSolutionv2isaninvitrodiagnostictestintendedforsequencing-basedscreeningforthe.
VeriSeq NIPT Solution v2 Package Insert 200006957 v00 for Canada. Illumina has launched the VeriSeq NIPT Solution v2 a CE-IVD next-generation sequencing-based approach to noninvasive prenatal testing. VeriSeq NIPT Solution v2 provides accurate information about fetal chromosomal status as early as 10 weeks of gestation using a single maternal blood draw.
The VeriSeq NIPT Solution v2 is an in vitro diagnostic test intended for use as a screening test for the detection of genome-wide fetal genetic anomalies from maternal peripheral whole blood specimens in pregnant women of at least 10 weeks gestation. The VeriSeq NIPT Microlab STAR allows a single user to prepare and analyze 48 or 96 samples simultaneously with results in approximately one day compared to two days or longer using other methods. VeriSeq NIPT Solution v2.
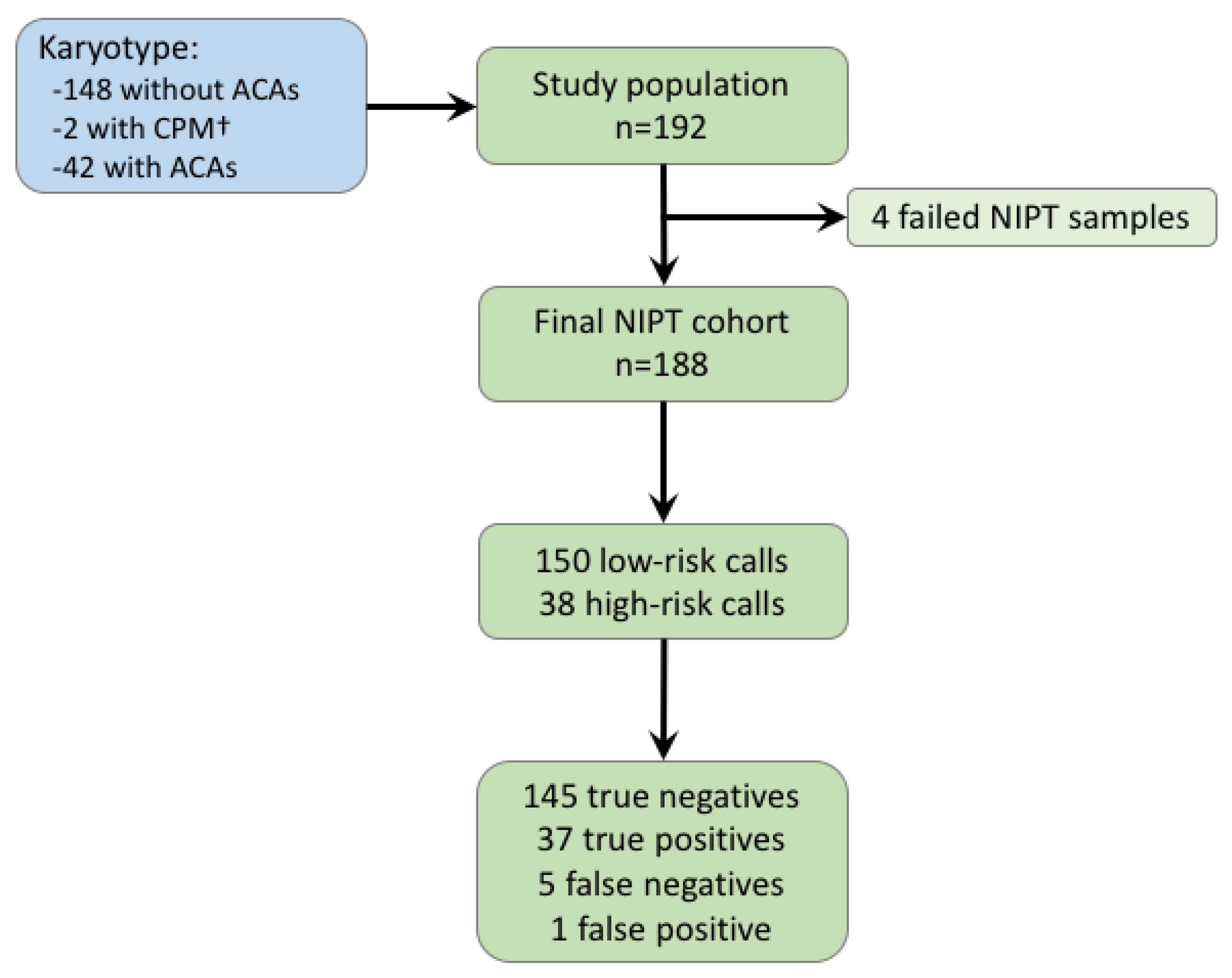
Jcm Free Full Text Strategy For Use Of Genome Wide Non Invasive Prenatal Testing For Rare Autosomal Aneuploidies And Unbalanced Structural Chromosomal Anomalies Html

Illumina On Twitter Fdesouza Version 2 Of Veriseq Nipt Will Ship In 1h 2019 Adding Karyotype Resolution Across The Genome And Increasing The Number Of Genetic Diseases That Can Be Detected Jpm19

Illumina Next Generation Genomic Introduce Veriseq Nipt Solution
Veriseq Pgs Kit Miseq Vitrolife

In Lab Screening With Nipt Turnkey Sample To Results In Your Lab

Illumina S Noninvasive Prenatal Screening Kit Receives Regulatory Approval In S Korea Business Wire

Distribution Of Fetal Fraction For Samples That Underwent Genome Wide Download Scientific Diagram
5524澳门24小时线路 5524澳门24小时线路网站 主頁 欢迎您

Veriseq Nipt Solution V2 Comprehensive And Reliable Nipt Solution

The Veriseq Nipt Solution Youtube
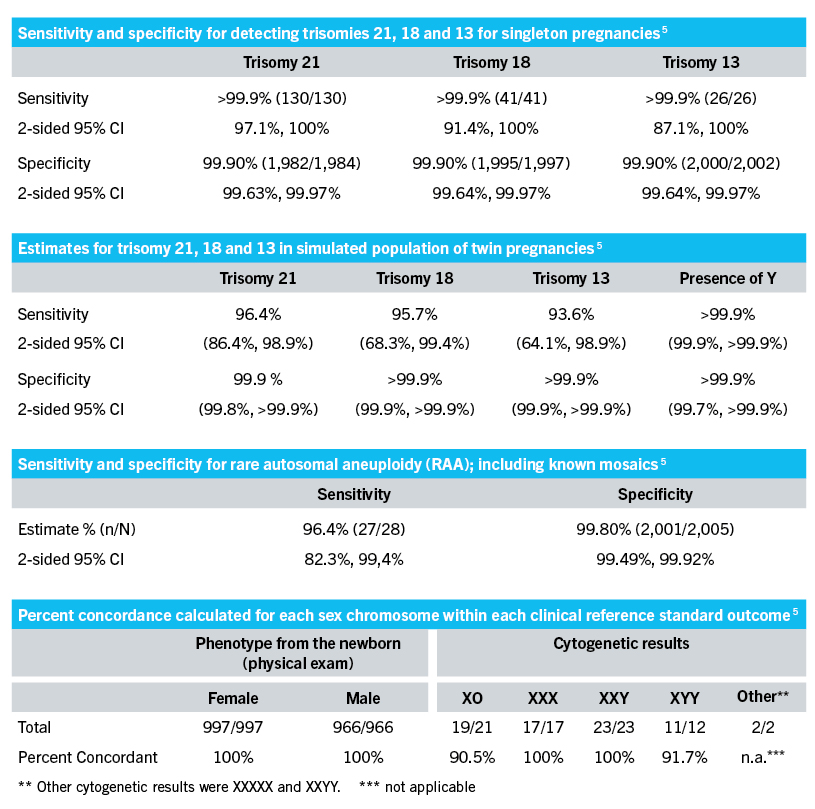
Performance Qualification Praenatest
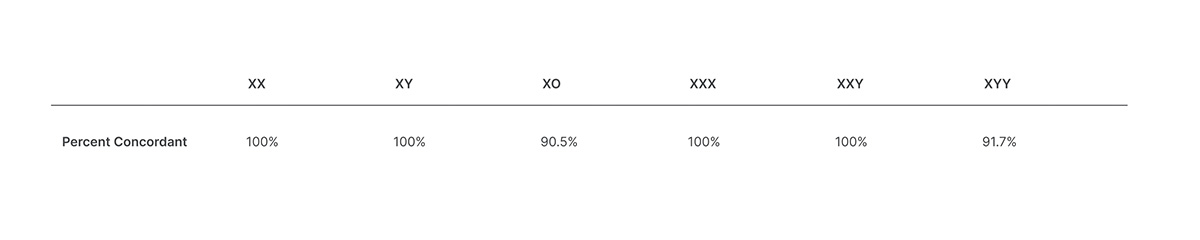
Veriseq Nipt Solution V2 Comprehensive And Reliable Nipt Solution
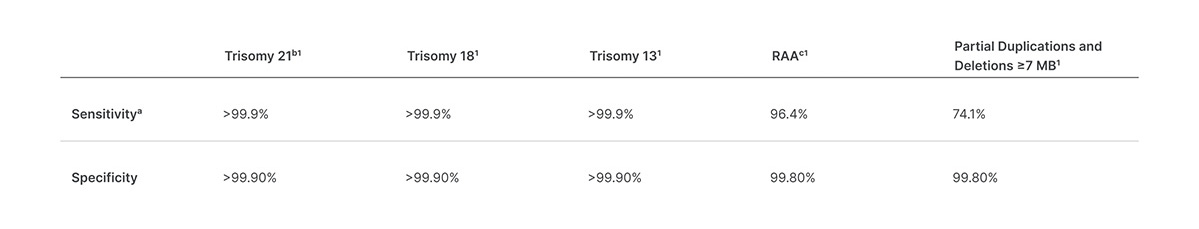
Veriseq Nipt Solution V2 Comprehensive And Reliable Nipt Solution

Veriseq Nipt Solution V2 Support

Noninvasive Prenatal Testing How Far Can We Reach Detecting Fetal Copy Number Variations European Journal Of Obstetrics And Gynecology And Reproductive Biology
Veriseq Nipt Solution V2 Comprehensive And Reliable Nipt Solution

Illumina Introduces Expanded Version Of Veriseq Nipt Solution Offering More Comprehensive Detection Of Rare Chromosomal Conditions Business Wire

In Lab Screening With Nipt Turnkey Sample To Results In Your Lab
0 Response to "veriseq nipt v2"
Post a Comment